Man receives world’s first melanoma vaccine
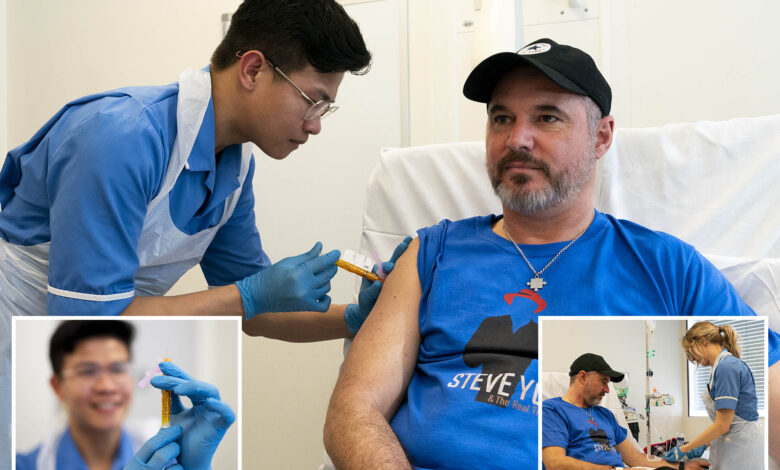
A trial for the world’s first-ever mRNA cancer vaccine for melanoma is underway in the UK.
Melanoma is the deadliest form of skin cancer. Around 100,000 Americans are expected to be diagnosed with melanoma in 2024, according to the American Cancer Society.
Hundreds of patients are currently testing the vaccines, which are custom-made for each person, and are designed to tell their body to find cancer cells and prevent the disease from ever coming back. The vaccine is currently in Phase 3 trials and the research is being led by University College London Hospitals NHS Foundation Trust, the Guardian reported.
“This is one of the most exciting things we’ve seen in a really long time, It is absolutely custom-built for the patient – you couldn’t give this to the next patient in the line because you wouldn’t expect it to work,” UCLH researcher Dr. Heather Shaw told the BBC.
“It’s truly personalized. These things are hugely technical and finely generated for the patient,” she added.
Steven Young, 52, from Stevenage, Herts, England is one of the first patients to try the vaccine, the BBC reported.
He had a melanoma growth cut out of his scalp last August and he hopes that after getting the vaccine, his cancer will not return.
“[The trial] gave me a chance to feel like I was actually doing something to fight a potential unseen enemy. Scans showed I was radiologically clear, obviously there is still the chance I had cancer cells floating around undetected,” Young told BBC 4 Radio For Today Program.
“So rather than just sit there and wait and hope it was never going to come back, I actually had this chance to get involved in putting on some boxing gloves and squaring up to it,” he added.
Patients in the international trial must have had a melanoma surgically removed in the last 12 weeks for the best results. Some of the participants will get a placebo shot though none will know what they are receiving.
Doctors are giving patients the vaccine in tandem with either pembrolizumab or Keytruda, drugs that aid the immune system in killing cancer cells.
A Phase 2 trial published in December found that the vaccines greatly reduced the risk of melanoma coming back in cancer patients. The Phase 3 trial hopes to recruit 1,100 people.
The vaccine, called mRNA-4157 (V940) works similarly to the way the COVID-19 vaccine does. The vaccine matches the genetic signature of the patient’s body. It then tells the body to create proteins or antibodies to attack antigens and markers on the patient’s cancer cells.
The jab, manufactured by Moderna and Merck Sharp and Dohme is not yet available outside the clinical trials.